A recent US multi-center study involving over 1,400 patients with multiple sclerosis (MS) has found that those treated with rituximab experienced significantly higher rates of hospitalizations, infections, and hypogammaglobulinemia compared to patients on ocrelizumab. The study analyzed data from UCSF and a larger University of California–wide cohort over a four-year follow-up period.
At UCSF, 18.6% of rituximab-treated patients experienced at least one hospitalization, compared to 8.8% of those on ocrelizumab. In the UC-wide validation cohort, the difference was even more pronounced—35.3% of rituximab patients were hospitalized versus 12.2% of those on ocrelizumab. Hospitalization rates related to infections were also markedly higher in the rituximab group, including significantly increased cases of urinary tract infections, pneumonia, cellulitis, and vaginitis.
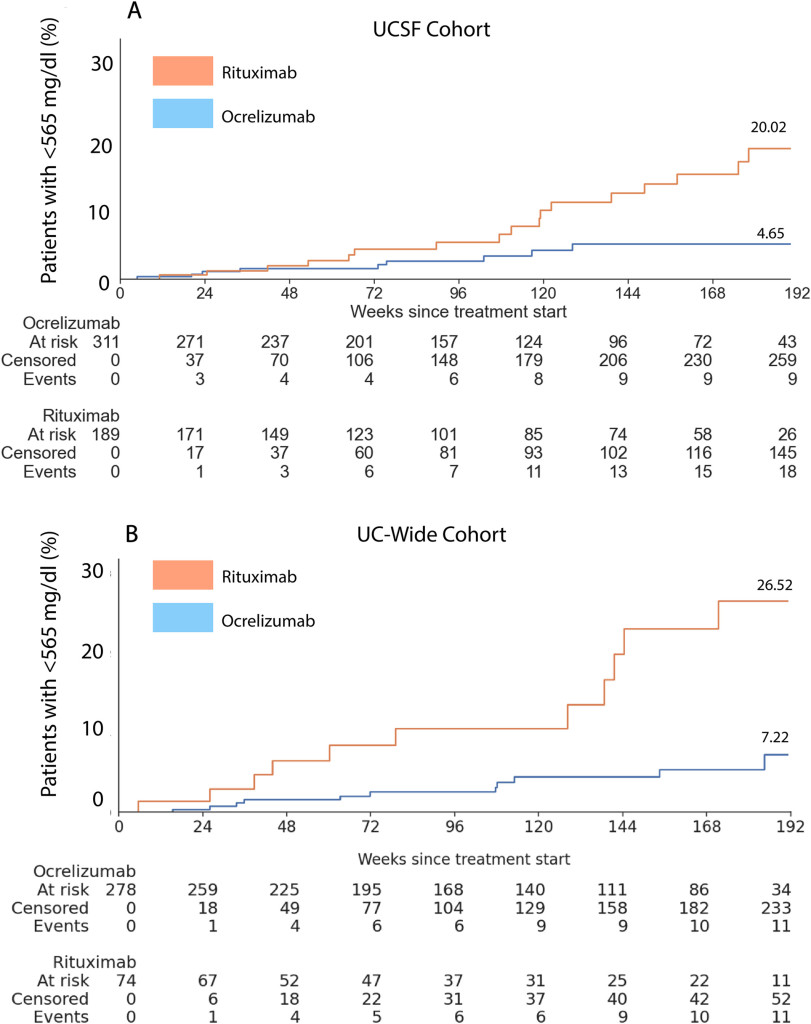
The risk of developing hypogammaglobulinemia was also greater among rituximab patients, with a 2 to 4-fold higher likelihood of low serum IgG levels (see Figure below). These patients were also more likely to require intravenous immunoglobulin (IVIg) treatment.
Even after adjusting for dosage, treatment duration, and potential selection bias, the differences in hospitalization and infection rates remained significant.

While there was a trend toward greater disability progression in the rituximab group, this finding was not statistically significant. Still, the overall data suggest that ocrelizumab may offer a more favorable safety profile than rituximab for long-term MS management, especially in terms of infection-related outcomes. I did wander if a similar comparison was performed in the Scandinavian countries, you’d get the same data.
Abstract
Ann Neurol. 2025 Sep 8.
Comparative Safety Profiles of Ocrelizumab and Rituximab in Multiple Sclerosis Treatment Using Real-World Evidence
Gabriel Cerono , Bruce A C Cree , Stephen L Hauser , Sergio E Baranzini
Objective: The objective of this study was to compare the long-term safety profiles of ocrelizumab and rituximab in persons with multiple sclerosis (MS).
Methods: Using retrospective data from the University of California (UC) Health System, we simulated a target clinical trial. The primary cohort from UC San Francisco (UCSF) and a validation cohort from 5 other UC Medical Centers were analyzed. After applying exclusion criteria and propensity score matching based on disease characteristics, demographics, and socioeconomic factors, we compared UCSF patients receiving ocrelizumab (n = 542) and rituximab (n = 271)and validated in the UC-wide MS population (n = 486 and n = 162 patients, respectively). The primary outcome was an all-cause hospitalization rate; secondary outcomes included hypogammaglobulinemia development and infection incidence.
Results: Rituximab showed higher all-cause hospitalization rates compared to ocrelizumab in both UCSF (incidence rate ratio [IRR] = 2.29, 95% confidence interval [CI] = 1.37-3.82, p = 0.001) and UC-wide cohorts (IRR = 4.54, 95% CI = 4.30-7.61, p < 0.001). Cumulative hazard ratios (HRs) were similarly elevated with rituximab at UCSF (HR = 2.27, 95% CI = 1.37-3.75, p = 0.001) and UC-wide (HR = 4.01, 95% CI = 2.25-6.32, p < 0.001). The risk of developing hypogammaglobulinemia was higher with rituximab at both UCSF (HR = 2.72, 95% CI = 1.18-6.29, p = 0.003) and UC-wide (HR = 4.79, 95% CI = 2.04-11.25, p < 0.001).
Interpretation: A more favorable safety profile was observed for ocrelizumab, with lower rates of hospitalization and hypogammaglobulinemia across 2 independent cohorts. These findings may help guide treatment strategies in persons with MS
Source: multiple-sclerosis-research.org