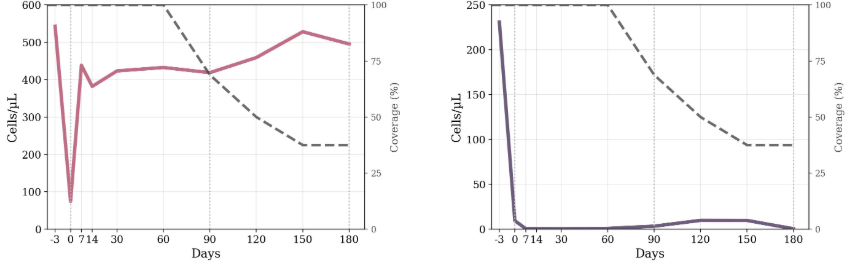
The answer to the question above is no, but they all deplete B cells…this study looks at T and B cells after depletion with ublituximab and T cells drop transiently naive B cells show rapid and long term depletion.
The depletion reproduced from paper below for CD8 T cells (left) AND B CELLS under creative common CC-BY. T cells are transiently reduced…so we think this is why ublituximab works or do you take the easier view that it has something to do with B cells.

In the blood naive cells make up about 60% of the cells and memory B cells about 30-35%. In the methods there is no mention of subdivision of the CD19+ B cells and why they are naive B cells and not a mix of naive and memory B cells.
However they have to say “However, anti-CD20 therapies also target a small subset of CD20+ T cells, which display heightened pro-inflammatory and CNS-migratory properties compared to CD20− counterparts. Their selective and more durable depletion may partly explain early clinical effects and immune resetting.”…
Zanghì A, Di Filippo PS, Papale M, Rutigliano C, Moretti MC, Avolio C, Corso G, D’Amico E. Modeling lymphocyte subset dynamics after ublituximab therapy in patients with multiple sclerosis: an Italian prospective study. Front Immunol. 2025 Nov 21;16:1688090.
Background and objectives: Ublituximab, a novel, glycoengineered anti-CD20 monoclonal antibody, has recently entered clinical use for multiple sclerosis (MS). In this study, we aimed to delineate the longitudinal kinetics of circulating lymphocyte subsets over the first 6 months following ublituximab initiation. Secondarily, we aimed to investigate whether relevant baseline demographic and clinical characteristics predicted the residual counts at day 30 after infusion of CD3+CD8+ T cells and CD19+ B naive cells, the two subsets that exhibited the most distinctive early kinetics.
Methods: A real-world prospective study was performed at the MS Center of Foggia, Italy. Inclusion criteria were patients with a diagnosis of relapsing MS who started ublituximab between 1 December 2024 and 31 May 2025. Longitudinal trajectories were modeled with subject-specific random-intercept linear mixed-effects models. To identify determinants of early residual depletion, linear regression models were built.
Results: A total cohort of 16 patients was enrolled, with a median age of 47 (Q1-Q3 41-58), 69% men, median EDSS of 4.5, and median body mass index (BMI) of 26.9 kg/m2. Mixed-effects models showed a significant effect of time on all lymphocyte subsets. CD3+ T cells decreased by 1,577 cells/μL immediately after ublituximab infusion (p < 0.001), returning to baseline from day 7 onward. CD3+CD8+ T cells dropped by approximately 400 cells/μL within the first week (day 7 = 44 cells/μL; 95% CI 11-77) and stabilized from day 30. CD3+CD4+ T cells fell by 1,133 cells/μL post-infusion (p < 0.001), but rebounded from day 7 and remained stable through day 180. CD19+ naive B cells remained profoundly suppressed throughout the 6 months (all p < 0.001). CD16+CD56+ NK cells showed a transient reduction of 239 cells/μL at day 0 (p = 0.004), normalizing by day 7. Regression analyses at day 30 indicate no significant baseline predictors for CD3+CD8+ T or CD19+ naive B-cell recovery (R² = 0.48 and 0.24, all p > 0.05). Infusion reactions were mild and self-limited; no adverse events occurred.
Discussion: In our cohort, ublituximab induced rapid, durable CD19+ naive B-cell depletion with only transient, reversible effects on other lymphocyte subsets and preserved immunoglobulin levels. This signature extends to older and high BMI patients, supporting ublituximab as a versatile therapeutic option across heterogeneous MS populations.
Source: multiple-sclerosis-research.org