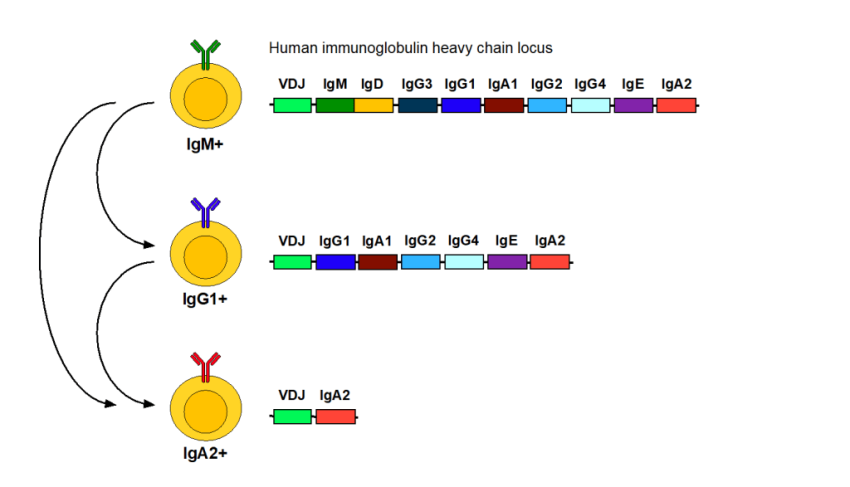
Infusion reactions can occur as a consequence of different things but one potential is an allergic reaction due to anti-drug antibodies. Now you have been told they are rare and it is often implied that they are unimportant. Whilst at the population level, this may be the case, for individuals with the problem, they can be very important. When antibodies are made they often start as IgM and then they class switch to IgG and with severe allergies there is a class switch to IgE. This sits on mast cells lining the skin and the gut and lungs and when stimulated they release histamine (makes things itchy) and other mediators and this can cause your airways to close which is very danagerous. You can see how the genes are structured the class switch (Tail effector end of the antibody) splices out the IgMIgD part and the variable bit may switch to IgG1..with alot of stimulation it could splited out the IgG1, IgA1, IgG2, IgG4 and become an IgE and could splice to become an IgA2. So IgE appear when IgG cells are stimulated alot.
Now whilst some anti-CD20 can induce anti-drug antibodies in about 80% of people in the summary of product characterisitics it says about only 1% of people may develop anti-drug antibodies, with ofatumumab it is even less (about 0.2%).

In this study they look to see how many people have IgE and there are 1.6% of people. with IgE so more people with anti-IgG. Anyway they switched to ofatumumab. This is not a bad choice as being fully human ofatumumab is differnt from ocrelizumab in the target binding regions it also binds to a different bit of CD20. However ocrelizumab shares some sequence with other anti-CD20 so if antibodies bind to that bit they could cross react and the problem wouldnt disappear
Bianco A, Aruanno A, Lucchini M, Cicia A, Longhino D, Bisurgi M, Colò F, Carlomagno V, Rizzi A, Buonomo A, Mirabella M. IgE-mediated allergic reactions to ocrelizumab in multiple sclerosis: A retrospective cohort study. Mult Scler Relat Disord. 2025 Oct 31;104:106826.
Background: Ocrelizumab, a humanized anti-CD20 monoclonal antibody, is widely used in multiple sclerosis (MS). Infusion-related reactions (IRRs) are common, while IgE-mediated allergic reactions are rare and poorly characterized.
Objectives: To evaluate the incidence and risk factors of IgE-mediated allergic reactions to ocrelizumab in MS patients.
Methods: We retrospectively analyzed MS patients treated with ocrelizumab (2018-2024) at a single center. Severe IRRs (grade ≥3) underwent allergologic assessment, including skin prick tests (SPT) and intradermal tests (IDT). Predictors of IgE-mediated reactions were explored.
Results: Among 387 patients, 94 (24.3%) developed IRRs; 19 (4.9%) were grade 3. Six cases (1.6%) were classified as IgE-mediated based on positive IDT and clinical presentation; all had negative SPTs. A history of allergic disease was more frequent among IgE-positive patients (66.7% vs. 26.5%, p=0.028). Allergy history (OR 11.5, p=0.009) and fewer ocrelizumab infusions (OR 0.60, p=0.047) were independent risk factors. Switching to ofatumumab was well tolerated in five out of six.
Conclusions: IgE-mediated allergic reactions to ocrelizumab are uncommon but important to recognize. IDT with immediate reading is useful for diagnosis. A history of allergy may help identify high-risk patients. Ofatumumab is a safe alternative in confirmed cases.
Source: multiple-sclerosis-research.org