Saving time and money is the usual saying and is the mantra for NICE. saving time means lower staff costs and saves money..For pharma by the NHS saving time they make more money. Why because their more expensive patented compound is used over cheaper genetics consuming more staff time.
As patents run out pharma has developed ways to cycle their parents to keep making money off the originator compound. They tweak the product to make it better than the originator compound and then tell the neuros or NICE why it is better. Neuros write papers on the cost saving.
The neuros switch drug and the companies are happy. You are happy as it is more convenient. It happens all the time. Ocrelizumab infused over hours compared to 10 minutes under the skin….No brainer where this one leads?.
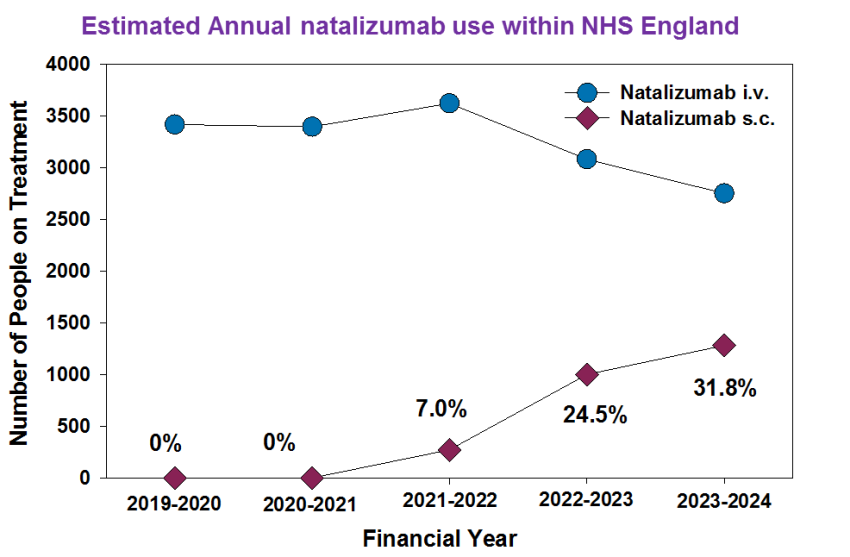
Similarly it has happened for natalizumab. As can be seen here given under the skin saves quality time….Here is the evidence of time saved. During COVID-19 under the skin ( sub (under) cutaneous (skin) was a favoured route because it avoided time interacting with people who may be infected, remembering that hospitals were full of people kill who were infected.
Anyway the marketing has worked and using public data available in England this can be seen. The infusion is 15ml or drug versus 2ml of under the skin drug so one person a year is about 26 ml which is 2ml x 13 (13 four week months a year) the infusion is 195ml (15ml x 13). As the amount of drug obtained by each pharmacy each month is public knowledge you can estimate the number of people recieving each formulation variant of each NHS trust once you know the code for the hospital.
It gives an estimate of people treated. But it is an under estimate because not everyone gets dosed at 4 week intervals and not everyone is dosed for the year, but the data clearly shows change it shows some places don’t use it… They may not have infusion chair capacity or infusions maybe given at a hub.

Data from Secondary Care medicines database.
There is perhaps a down side of under the skin delivery. As the same amount is given. Less therapeutic will reach the blood. On the whole this does not matter to efficacy for most people. That you can get efficacy with a 6 week interval tells you this.
However, under the skin route is slightly more immunogenic than into the blood as it is a sensitising route. This means more potential to induce antibodies. Surprising based on published data when you switch from infusion to subcutaneous no (n=0/136 Trojano et al. 2021) people were reported to make anti-drug antibodies. How could this be?.
In the trial data label it says 9-10 percent of people make anti-drug antibodies and 6 percent of people make persistent anti drug antibodies. So by being on infusion first I guess those destined to make antibodies have been weeded out and those left have been tolerant to drug This is a fudge due to the fact that only those that have the anti drug antibodies above a level where it can stop the drug working are reported meaning that 60-70 percent of people who did make an anti-drug response are not reported. This is also enhanced by not looking the anti drug antibodies for months after starting treatment at a time when they are wanting.
What happens when you start on natalizumab under the skin well the trial was very small they only reported 1 of 26 people (about 4%) which is about the same as the infusion. However in the label it is clearer this one person made persistent anti drug antibodies there were another 5 where it wasn’t persistent so using the fudge level under the skin induced anti drug antibodies in 23.1% percent so more than the 9.1% (n= 57/625 Polman et al. 2006), so this fits with the idea of immunogenicity potential…..Ester will soon tell us how you can get a sense of if this happening.
De Cock E, Jomaa K, Menon J, Acosta C, Esquejo-Leon R, Oliver-Smith P. Time and Motion Study to Quantify Time for Tysabri (Natalizumab) Intravenous Versus Subcutaneous to Treat Relapsing-Remitting Multiple Sclerosis in France, Spain, and the United Kingdom. Neurol Ther. 2026 . doi: 10.1007/s40120-026-00888-1
Introduction: Tysabri (natalizumab) is a recombinant humanized IgG4 monoclonal antibody for the treatment of adults with highly active relapsing-remitting multiple sclerosis (RRMS). A subcutaneous (SC) formulation administered by healthcare professionals (HCPs) is expected to save time for patients and healthcare staff compared with intravenous (IV) delivery. This observational study quantifies HCP and patient time with natalizumab IV vs natalizumab SC in patients with RRMS.
Methods: Seven sites across France (n = 3), Spain (n = 3), and the United Kingdom (UK) (n = 1) participated in this study. Primary endpoints were active HCP time for tasks related to preparation and administration processes of natalizumab, all tasks combined, and time in the infusion chair. The target sample was 15 observations each of natalizumab IV and SC per site. Results were extrapolated per patient per year. HCP satisfaction and preference were assessed via a one-time survey.
Results: A total of 213 observations were collected (102 IV and 111 SC). Mean total active HCP time (min) per visit (and annually) was 15.8 (205.8) for IV and 9.1 (118.4) for SC (- 42.5% [pooled], – 29.3% [Spain], – 48.1% [France], and – 56.5% [UK]). Mean time in the infusion chair (min) was 95.2 for IV and 33.5 for SC (- 64.9% [pooled], – 60.4% [Spain], – 67.3% [France], and – 69.2% [UK]). Mean HCP satisfaction score for administration was higher for SC than for IV (9.2 vs 8.2; p = 0.026). Seventy percent of HCPs stated a preference for SC, of which 56.3% stated a very strong and 18.8% a fairly strong preference.
Conclusions: Natalizumab SC offers substantial savings in active HCP time and patient chair time compared with natalizumab IV. HCPs reported higher satisfaction and a preference for natalizumab SC. The HCP time and infusion chair capacity made available with natalizumab SC could be reallocated to other patient-care activities or used to treat additional patients, thereby improving overall healthcare efficiency.
COI Multiple
Diclaimer: My views alone.
Source: multiple-sclerosis-research.org